The Hidden Carbon Cost of Herbicides: Emissions from Product Manufacturing

When you grab herbicide, you're likely focused on weed control or habitat restoration. It feels like a positive step, right? But what if that bottle has a secret identity, linking it directly to fossil fuels and significant carbon emissions?
Many common herbicides, including glyphosate, are not just chemicals. They are, at their core, refined petroleum products. Pouring glyphosate into a sprayer is, in a way, like pouring out a carefully processed version of crude oil, carrying a larger environmental footprint than we realize.

From Oil to Weed Killer: A Complex Journey
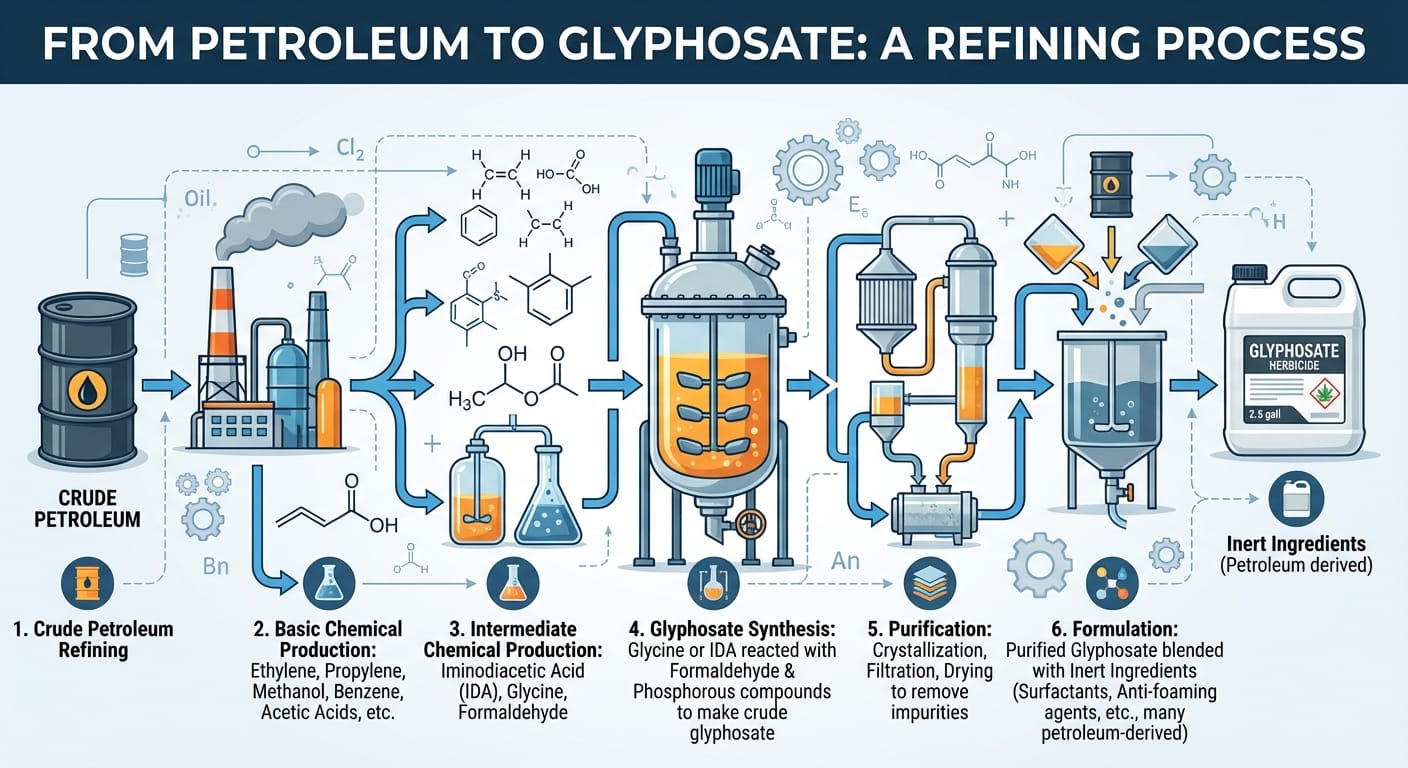
Turning crude petroleum into glyphosate is an intricate process, more demanding than making car fuel. A 2.5-gallon container of herbicide involves multiple intensive chemical refining steps, each adding to its carbon footprint.
It starts with crude petroleum refining, then basic chemical production breaks it into building blocks like ethylene and methanol. Next, intermediate chemical production creates compounds such as iminodiacetic acid (IDA) and formaldehyde.
These are combined in glyphosate synthesis, where IDA reacts with formaldehyde and phosphorous compounds to form "crude glyphosate." This crude form then undergoes extensive purification through crystallization, filtration, and drying to remove impurities.
Finally, the purified glyphosate is blended in the formulation step with "inert" ingredients like surfactants. Many of these "inert" components also come from petroleum or natural gas, further tying the product to fossil fuels. Every manufacturing step is energy-intensive, producing waste and toxic by-products, all contributing to the product's "embodied carbon."
The Carbon Tally: Numbers You Might Not Expect
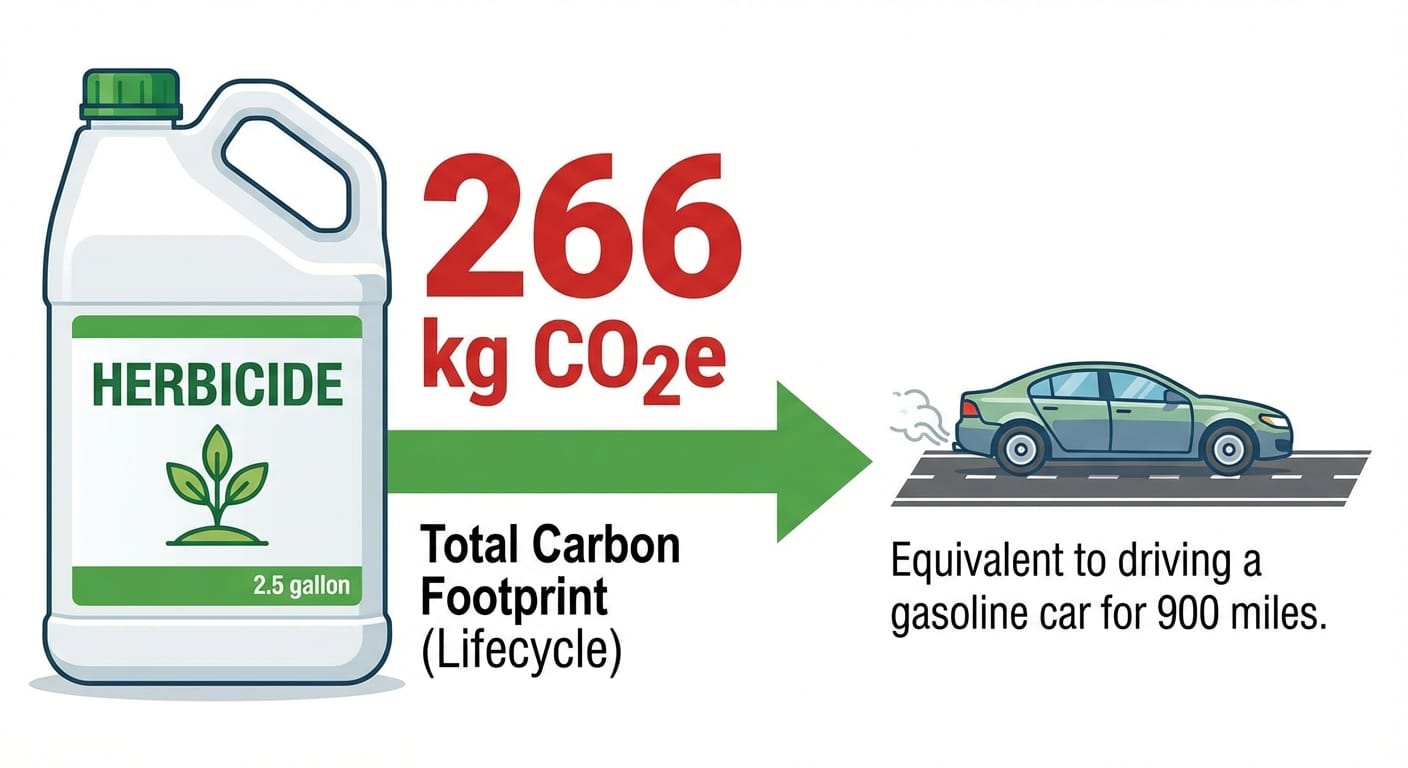
A 2009 study estimated each kilogram of purified glyphosate is responsible for 31.29 kg of CO2 equivalent emissions. For a typical 2.5-gallon container of glyphosate products (53.8% active ingredients), let's break it down.
A 2.5-gallon container weighs about 27 pounds. With 53.8% active ingredient, that's 14.53 lbs of active ingredient, or 6.59 kg. The active ingredient alone contributes about 206 kg of CO2 (6.59 kg AI * 31.29 kg CO2e/kg AI). Add an estimated 60 kg of CO2 for "inert" ingredients, totaling approximately 266 kilograms of CO2 emissions for one bottle.
To put that in perspective:
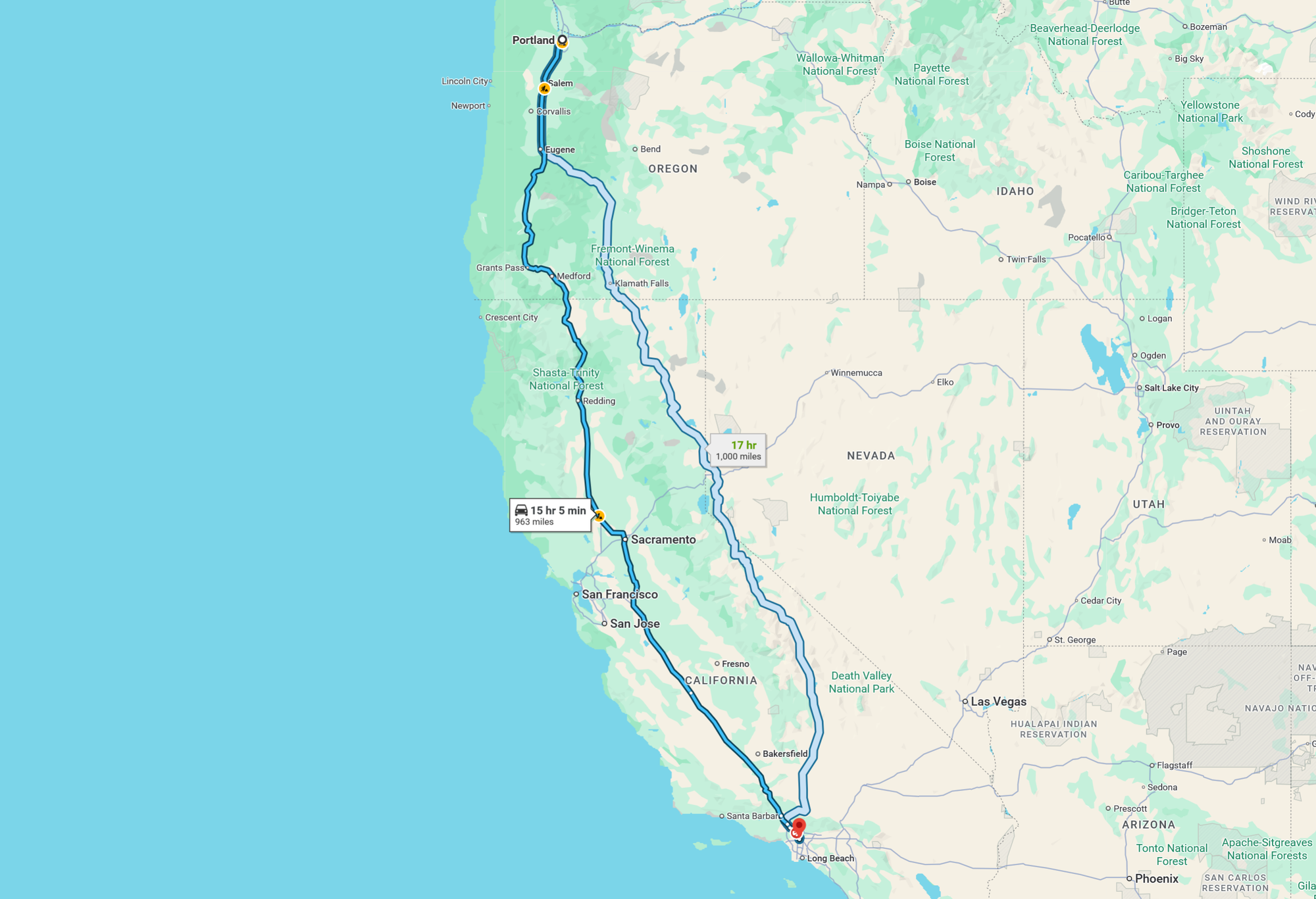
Manufacturing one 2.5-gallon container of glyphosate produces roughly the same emissions as burning 30 gallons of gas or driving 900 miles from Portland, Oregon to San Francisco in the average passenger car.
That's a long road trip! This analogy helps visualize the substantial, often unseen emissions tied to herbicide production.
Beyond Manufacturing: The Full Picture
The environmental impact goes beyond the factory. Emissions also arise from packaging, transporting these liquids, and even the application process itself (e.g., fuel for sprayers).
Once in the environment, the chemical degrades, releasing more carbon into the soil. Herbicide use can also harm soil biology and organic matter. Healthy soil is a vital "carbon sink." When herbicides compromise this, it reduces carbon sequestration, potentially leading to more atmospheric emissions.
Rethinking Our Approach to Restoration
Our goals in habitat restoration are positive: remove invasives, foster native growth. But considering herbicides are petroleum products with a significant carbon footprint, how should we weigh their use?
Herbicides can be necessary tools. But understanding their full "embodied carbon emissions" empowers us to make informed choices. We should strive for responsible use, minimizing reliance, and ensuring a project's total emissions don't negate its positive environmental benefits. Our aim is a truly net positive impact.
Sources: "Estimation of the greenhouse gas emissions from agricultural pesticide manufacture and use" Audrey, et al (2009) https://dspace.lib.cranfield.ac.uk/server/api/core/bitstreams/78cd42ff-e564-4112-ab50-e5e7fc4da80a/content

Member discussion